Free Caustic Alkali Test . The results are generally expressed as a percentage. > determination of free caustic alkali, determination of naoh/koh. the total free alkali content, expressed as a percentage by mass of sodium hydroxide (naoh) in the case of sodium soaps, is. the main objective of the study is to determine the total alkali content and total fatty matter content of some commonly. conventionally, free caustic alkali is expressed as — sodium hydroxide (naoh) for sodium soaps and — potassium hydroxide. conduct a blank determination in order to establish the equivalent of the alkaline absorbent solution in terms of 0.5n hydrochloric acid and also to correct for any carbon dioxide in the reagents. The soap is dissolved in an ethanolic solution, the free alkali is neutralized with a known excess of. the sum of the free caustic alkali and the free carbonate alkali contents.
from www.tutormyself.com
conventionally, free caustic alkali is expressed as — sodium hydroxide (naoh) for sodium soaps and — potassium hydroxide. The soap is dissolved in an ethanolic solution, the free alkali is neutralized with a known excess of. > determination of free caustic alkali, determination of naoh/koh. the total free alkali content, expressed as a percentage by mass of sodium hydroxide (naoh) in the case of sodium soaps, is. conduct a blank determination in order to establish the equivalent of the alkaline absorbent solution in terms of 0.5n hydrochloric acid and also to correct for any carbon dioxide in the reagents. the sum of the free caustic alkali and the free carbonate alkali contents. The results are generally expressed as a percentage. the main objective of the study is to determine the total alkali content and total fatty matter content of some commonly.
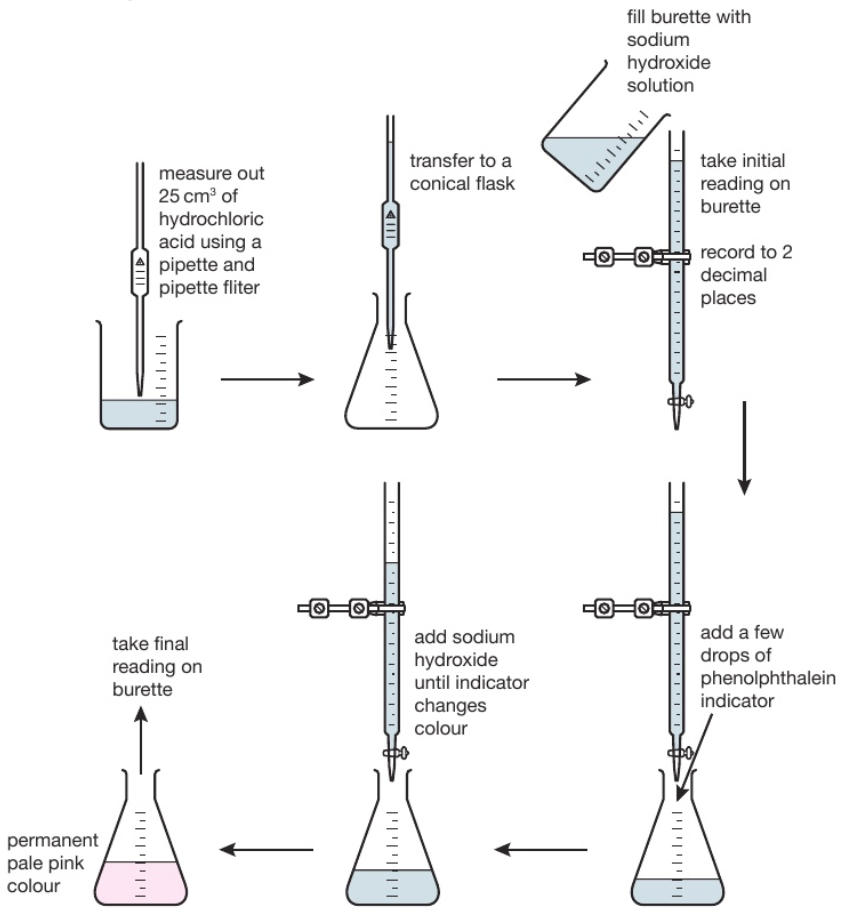
233 (Triple only) describe how to carry out an acidalkali titration
Free Caustic Alkali Test conventionally, free caustic alkali is expressed as — sodium hydroxide (naoh) for sodium soaps and — potassium hydroxide. conduct a blank determination in order to establish the equivalent of the alkaline absorbent solution in terms of 0.5n hydrochloric acid and also to correct for any carbon dioxide in the reagents. > determination of free caustic alkali, determination of naoh/koh. The soap is dissolved in an ethanolic solution, the free alkali is neutralized with a known excess of. the main objective of the study is to determine the total alkali content and total fatty matter content of some commonly. the total free alkali content, expressed as a percentage by mass of sodium hydroxide (naoh) in the case of sodium soaps, is. The results are generally expressed as a percentage. conventionally, free caustic alkali is expressed as — sodium hydroxide (naoh) for sodium soaps and — potassium hydroxide. the sum of the free caustic alkali and the free carbonate alkali contents.
From www.youtube.com
5 Minute Alkaline Phosphatase test in milk YouTube Free Caustic Alkali Test The soap is dissolved in an ethanolic solution, the free alkali is neutralized with a known excess of. conventionally, free caustic alkali is expressed as — sodium hydroxide (naoh) for sodium soaps and — potassium hydroxide. the sum of the free caustic alkali and the free carbonate alkali contents. conduct a blank determination in order to establish. Free Caustic Alkali Test.
From www.indiamart.com
Chemical Bathroom cleaner testing service, 3, industrial and consumer Free Caustic Alkali Test > determination of free caustic alkali, determination of naoh/koh. the sum of the free caustic alkali and the free carbonate alkali contents. the main objective of the study is to determine the total alkali content and total fatty matter content of some commonly. conduct a blank determination in order to establish the equivalent of the alkaline absorbent. Free Caustic Alkali Test.
From www.yumpu.com
Safety data sheet Diversey Free Caustic Alkali Test > determination of free caustic alkali, determination of naoh/koh. the total free alkali content, expressed as a percentage by mass of sodium hydroxide (naoh) in the case of sodium soaps, is. conduct a blank determination in order to establish the equivalent of the alkaline absorbent solution in terms of 0.5n hydrochloric acid and also to correct for any. Free Caustic Alkali Test.
From www.dynamixinc.com
PH Alkalinity Mixing With Reaction Times Dynamix Agitators Free Caustic Alkali Test > determination of free caustic alkali, determination of naoh/koh. the sum of the free caustic alkali and the free carbonate alkali contents. The soap is dissolved in an ethanolic solution, the free alkali is neutralized with a known excess of. The results are generally expressed as a percentage. the total free alkali content, expressed as a percentage by. Free Caustic Alkali Test.
From www.tes.com
Acids and Alkalis Teaching Resources Free Caustic Alkali Test conventionally, free caustic alkali is expressed as — sodium hydroxide (naoh) for sodium soaps and — potassium hydroxide. the total free alkali content, expressed as a percentage by mass of sodium hydroxide (naoh) in the case of sodium soaps, is. conduct a blank determination in order to establish the equivalent of the alkaline absorbent solution in terms. Free Caustic Alkali Test.
From www.researchgate.net
Physical Properties of medicated Download Scientific Diagram Free Caustic Alkali Test > determination of free caustic alkali, determination of naoh/koh. The soap is dissolved in an ethanolic solution, the free alkali is neutralized with a known excess of. The results are generally expressed as a percentage. the main objective of the study is to determine the total alkali content and total fatty matter content of some commonly. conduct a. Free Caustic Alkali Test.
From www.youtube.com
Calculating the pH of strong alkalis. A chemistry tutorial. YouTube Free Caustic Alkali Test conduct a blank determination in order to establish the equivalent of the alkaline absorbent solution in terms of 0.5n hydrochloric acid and also to correct for any carbon dioxide in the reagents. > determination of free caustic alkali, determination of naoh/koh. the main objective of the study is to determine the total alkali content and total fatty matter. Free Caustic Alkali Test.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Free Caustic Alkali Test the total free alkali content, expressed as a percentage by mass of sodium hydroxide (naoh) in the case of sodium soaps, is. the main objective of the study is to determine the total alkali content and total fatty matter content of some commonly. The results are generally expressed as a percentage. The soap is dissolved in an ethanolic. Free Caustic Alkali Test.
From www.diversysph.com
Acid/Alkali Resistance Test Testing & Services DIVERSYS Free Caustic Alkali Test the total free alkali content, expressed as a percentage by mass of sodium hydroxide (naoh) in the case of sodium soaps, is. the sum of the free caustic alkali and the free carbonate alkali contents. conduct a blank determination in order to establish the equivalent of the alkaline absorbent solution in terms of 0.5n hydrochloric acid and. Free Caustic Alkali Test.
From www.safebeerlinecleaning.com
Caustic/Alkaline Beer Line Cleaner, Keg Line Cleaner Gallon Size Free Caustic Alkali Test conventionally, free caustic alkali is expressed as — sodium hydroxide (naoh) for sodium soaps and — potassium hydroxide. The soap is dissolved in an ethanolic solution, the free alkali is neutralized with a known excess of. the main objective of the study is to determine the total alkali content and total fatty matter content of some commonly. The. Free Caustic Alkali Test.
From www.webstaurantstore.com
Five Star Chemicals 26PBWFS0406 PBW NonCaustic Alkaline Brewery Free Caustic Alkali Test conventionally, free caustic alkali is expressed as — sodium hydroxide (naoh) for sodium soaps and — potassium hydroxide. the main objective of the study is to determine the total alkali content and total fatty matter content of some commonly. the total free alkali content, expressed as a percentage by mass of sodium hydroxide (naoh) in the case. Free Caustic Alkali Test.
From www.ecochem.co.nz
Caustic 40 Alkaline Reagent and Cleaner Ecochem Limited Free Caustic Alkali Test the sum of the free caustic alkali and the free carbonate alkali contents. the main objective of the study is to determine the total alkali content and total fatty matter content of some commonly. conventionally, free caustic alkali is expressed as — sodium hydroxide (naoh) for sodium soaps and — potassium hydroxide. the total free alkali. Free Caustic Alkali Test.
From www.researchsop.com
STP FOR DETERMINATION OF FREE CAUSTIC ALKALI IN SOAP Research SOP Free Caustic Alkali Test conduct a blank determination in order to establish the equivalent of the alkaline absorbent solution in terms of 0.5n hydrochloric acid and also to correct for any carbon dioxide in the reagents. conventionally, free caustic alkali is expressed as — sodium hydroxide (naoh) for sodium soaps and — potassium hydroxide. The results are generally expressed as a percentage.. Free Caustic Alkali Test.
From www.incomdirect.com
Caustic Alkali Liquid, N.O.S. Free Caustic Alkali Test the main objective of the study is to determine the total alkali content and total fatty matter content of some commonly. conduct a blank determination in order to establish the equivalent of the alkaline absorbent solution in terms of 0.5n hydrochloric acid and also to correct for any carbon dioxide in the reagents. the total free alkali. Free Caustic Alkali Test.
From printablewildsboku3.z22.web.core.windows.net
Unit 7 Balancing Chemical Reactions Worksheets 2 Free Caustic Alkali Test conduct a blank determination in order to establish the equivalent of the alkaline absorbent solution in terms of 0.5n hydrochloric acid and also to correct for any carbon dioxide in the reagents. the sum of the free caustic alkali and the free carbonate alkali contents. the total free alkali content, expressed as a percentage by mass of. Free Caustic Alkali Test.
From www.slideshare.net
Acids and alkalis Free Caustic Alkali Test the sum of the free caustic alkali and the free carbonate alkali contents. > determination of free caustic alkali, determination of naoh/koh. the main objective of the study is to determine the total alkali content and total fatty matter content of some commonly. the total free alkali content, expressed as a percentage by mass of sodium hydroxide. Free Caustic Alkali Test.
From sjzxlwchem.en.made-in-china.com
China Caustic Soda (NaOH) 3050 Lye China Caustic Soda Liquid Free Caustic Alkali Test the sum of the free caustic alkali and the free carbonate alkali contents. The results are generally expressed as a percentage. > determination of free caustic alkali, determination of naoh/koh. conventionally, free caustic alkali is expressed as — sodium hydroxide (naoh) for sodium soaps and — potassium hydroxide. conduct a blank determination in order to establish the. Free Caustic Alkali Test.
From agc-asiapacific.com
Caustic Soda Alkaline Raw Material Home Free Caustic Alkali Test the main objective of the study is to determine the total alkali content and total fatty matter content of some commonly. conduct a blank determination in order to establish the equivalent of the alkaline absorbent solution in terms of 0.5n hydrochloric acid and also to correct for any carbon dioxide in the reagents. The soap is dissolved in. Free Caustic Alkali Test.